Factors that influence enzyme activity
Enzymes are biological catalysts that make chemical reactions finish faster. These enzymes are affected by 5 factors which are: temperature, pH, concentration, inhibitors and water. In this essay we will explore the influence of each factor.
Temperature:
Starting with the first factor, temperature, depending on the type of protein the enzyme is formed, the optimal temperature can vary between 37 and 40 degrees Celsius [1]. Under extreme temperature, enzymes can lose activity but there are a few exceptions, for example TsLI isomerase works at above 95 °C [2].
However, some enzymes that come from hyperthermophilic organisms still work even at temperatures above 100 °C. These enzymes are very heat-stable because of several special features working together, not just one. Their stability comes from things like strong internal bonds that hold the protein together, tightly packed structures that make it harder to fall apart, and fewer flexible or loose parts that could break down at high temperatures. Some of these enzymes are made up of several connected subunits, which helps them stay stable when it’s really hot. Even though this rigid structure helps them survive heat, it also means they may not work as well at lower temperatures, since they can’t move as easily to carry out reactions. Because they can stay active at very high temperatures where normal enzymes would stop working, hyperthermophilic enzymes are useful in industries that run processes under extreme heat [3].
Some microorganisms, called psychrophiles, live in extremely cold places like the deep ocean or polar regions. They produce cold-active enzymes that can work well even at temperatures near freezing, while most normal enzymes would stop working. These enzymes are able to function in the cold because they have more flexible structures and weaker internal bonds, which let them move and react easily at low temperatures. However, this flexibility also means they can lose stability and stop working when it gets warmer. Cold-active enzymes are useful in many industries, such as food production, medicine, and environmental cleanup, because they work at low temperatures without needing extra heat. This saves energy and helps protect heat-sensitive materials. Scientists are now studying and improving these enzymes through protein engineering so they can be used more widely and effectively [4].
pH:
The next factor is pH, each enzyme works at around 7.5 [1]. If that pH isn’t reached, the enzyme won’t work with the best efficiency [1]. We have some exceptions here as well, Bifunctional Man/Cel5B has maximal activity at pH 5.5 and Nitphym who has a very large range [2]. Each enzyme has its own specific optimum pH, which can vary widely, some enzymes work best in very acidic conditions, around pH 2–3, while others prefer strongly basic conditions, up to pH 9–10. Changes in pH affect the charges on both the enzyme’s amino acid side chains and the substrate, which can change how well the substrate fits into the enzyme’s active site. Extreme pH levels can distort or even denature the enzyme’s three-dimensional structure, permanently destroying its activity. Enzymes that rely mainly on uncharged groups tend to have a broader pH range in which they can function, while enzymes that use charged groups have a narrower pH range and quickly lose activity if the pH shifts. For example, acid phosphatase works best at pH 3.8 and alkaline phosphatase at pH 9.5; although both are found in blood plasma, they are not active at the normal body pH of 7.4. In laboratory experiments, enzyme activity should always be measured at or near the optimum pH, because testing too far from that value gives inaccurate results. Small pH differences, such as ±0.1 units, usually do not significantly affect enzyme activity, but larger shifts can have a strong impact [5].
Concentration:
Now on the concentration factors, there are 2 types that can influence the reactions: substrate and enzyme [1]. If the substrate concentration rises, the reaction rate increases until it reaches a limit [1]. At this point, all enzyme active sites are occupied, meaning the enzymes are saturated [1]. If the enzyme concentration is boosted, the reaction rate is sped up as long as substrate is present to bind [1]. However, once all substrate molecules are occupied, adding more enzymes will have no further effect [1]. Extremozymes, on the other hand, are much more tolerant [2]. For example, the enzyme Nitphym nitrilase was shown to keep working even when exposed to very high substrate concentrations up to 500 mm of nitriles, a level that would normally deactivate many regular enzymes [2].
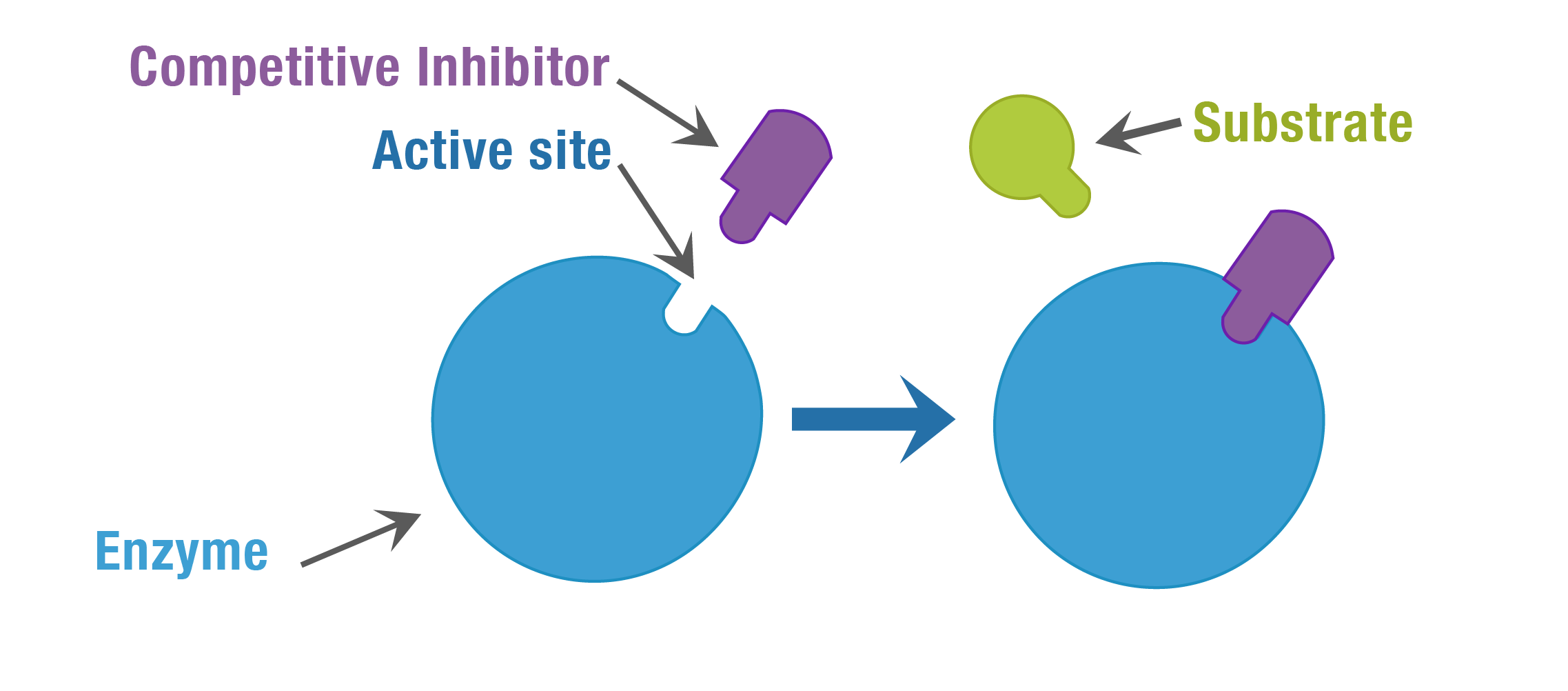
Inhibitors:
[7]
There are 2 types of inhibitors that can influence the function of the enzyme: competitive and non-competitive inhibitors [1]. Competitive inhibitors are when the substrate “looks like” the molecule, the inhibitors bind to the enzyme blocking of the substrate. Noncompetitive inhibitors are when the inhibitor enters the enzyme without blocking the substrate and changing the shape of the enzyme so that the substrate can no longer interact with it [7].
According to a research made by Sonia Del Prete and Pagano in 2024, enzyme inhibitors are substances that reduce or stop enzyme activity and can act reversibly or irreversibly. The reversible inhibitors bind temporarily to enzymes through weak interactions such as hydrogen bonds, ionic interactions or hydrophobic forces. Their effects can be reversed once the inhibitor detaches, allowing the enzyme to return to normal function. Some examples are alpha-glucosidase inhibitors which help controlling sugar in type 2 diabetes or carbonic anhydrase inhibitors which help lowering intraocular pressure or treat metabolic conditions. The irreversible inhibitors form covalent bonds with the enzyme permanently changing its structure making the enzyme inactive. Even if the inhibitor is removed, the enzyme will still remain inactive. Some examples are poisons and pesticides that target enzymes in humans or pets [6].
Water:
Enzymes need a certain level of hydration to become active [1]. They usually stop working or lose their shape when they are exposed to water [2]. This is because the solvents interfere with the weak bonds that hold the enzyme’s structure together, causing it to unfold and lose its activity [2].
In food preservation it is essential to fully suppress enzymatic activity when storage temperatures fall below the phase transition point [1].
In conclusion:
Enzymes are highly sensitive biological catalysts whose activity depends on several key factors which are temperature, pH, concentration, inhibitors and water. Each of these factors can increase or slow down enzyme performances depending on specific conditions or its type. Temperature and pH determine the enzyme’s structural stability and optimal working range, while concentration affect the rate of reaction. Inhibitors can regulate or completely block enzyme activity and water plays a crucial role in maintaining enzyme structure and function. Understanding how these factors interact with the enzyme is essential in biological researches and in practical applications in medicine and industry.
References (7):
1. Shrief, E. (2020). Factors Affecting Enzyme Activity. [online] ResearchGate. Available at: https://www.researchgate.net/publication/347439618_Factors_Affecting_Enzyme_Activity.
2. Mesbah, N.M. (2021). Editorial: Enzymes From Extreme Environments, Volume II. Frontiers in Bioengineering and Biotechnology, 9. doi:https://doi.org/10.3389/fbioe.2021.799426.
3. Unsworth, L.D., van der Oost, J. and Koutsopoulos, S. (2007). Hyperthermophilic enzymes−stability, activity and implementation strategies for high temperature applications. FEBS Journal, 274(16), pp.4044–4056. doi:https://doi.org/10.1111/j.1742-4658.2007.05954.x.
4. Hamid, B., Bashir, Z., Yatoo, A.M., Mohiddin, F., Majeed, N., Bansal, M., Poczai, P., Almalki, W.H., Sayyed, R.Z., Shati, A.A. and Alfaifi, M.Y. (2022). Cold-Active Enzymes and Their Potential Industrial Applications—A Review. Molecules, [online] 27(18), p.5885. doi:https://doi.org/10.3390/molecules27185885.
5. https://www.ucl.ac.uk/~ucbcdab/enzass/pH.htm
6. Sonia Del Prete and Pagano, M. (2024). Enzyme Inhibitors as Multifaceted Tools in Medicine and Agriculture. Molecules, [online] 29(18), pp.4314–4314. doi:https://doi.org/10.3390/molecules29184314.
7. Monash University (2025). Factors affecting enzyme activity. [online] Student Academic Success. Available at: https://www.monash.edu/student-academic-success/biology/regulation-of-biochemical-pathways/factors-affecting-enzyme-activity.