What is an Atom?
Everything around you, your phone, the air, the food is made up of atoms. Atoms are the smallest units of matter that still keep properties of an element. They are like the “LEGO bricks“ of the universe, and by joining together in different ways, they make every material we see.
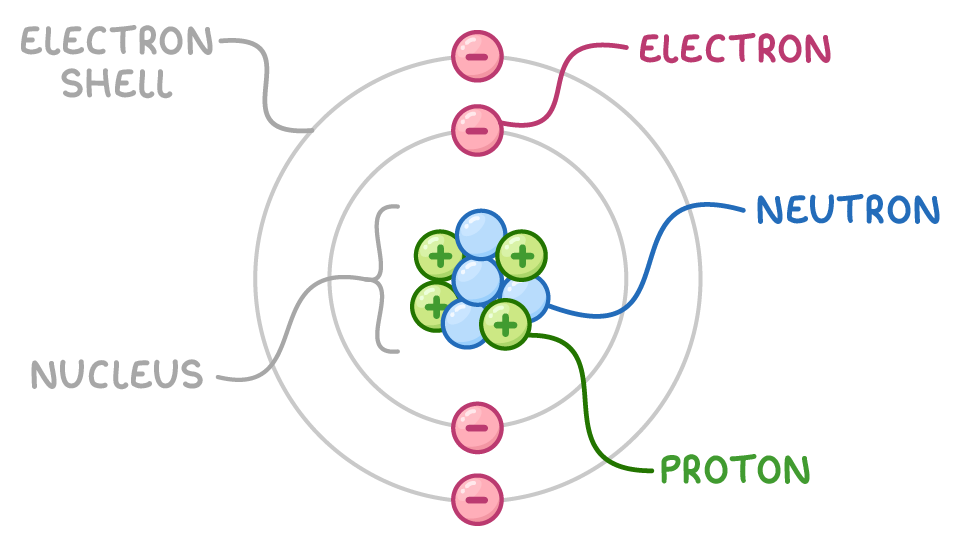
The Parts of an Atom
An atom isn’t just an empty ball, it has three main parts:
Protons: These are tiny particles with a positive charge. They sit in the middle of the atom, inside a dense center called the nucleus. The number of protons decides which element the atom is. For example, hydrogen has 1 proton, carbon has 6, and oxygen has 8.
Neutrons: These also live in the nucleus, but they carry no charge (they’re neutral). Neutrons help hold the nucleus together and give atoms different “versions,” called isotopes.
Electrons: These are much smaller and have a negative charge. They zoom around the nucleus in regions called shells or energy levels. Even though electrons are tiny, they are the ones that decide how atoms connect with each other.
If the atom were the size of a football stadium, the nucleus would be a small pea at the center, and the electrons would be buzzing around the outer seats.
Why Doesn’t the Atom Fall Apart?
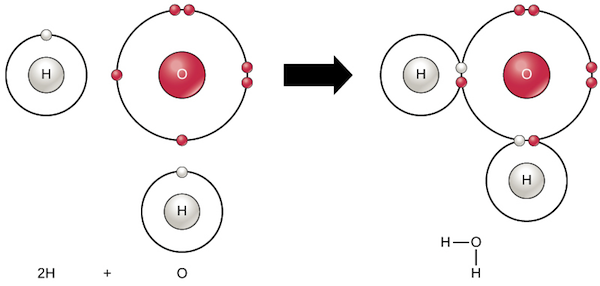
Diagram showing how water (H₂O) molecules form covalent bonding
You might wonder: if protons all have positive charges, why don’t they push each other away? The answer is a special force called the strong nuclear force. It’s much stronger than the repulsion between protons and keeps the nucleus tightly packed. Electrons, on the other hand, stay close to the nucleus because they are attracted to the positive charge of the protons.
How Atoms Combine
Atoms rarely exist alone. They like to join up and make molecules:
Diagram showing how table salt (NaCl) molecules form ionic bonding
Covalent bonds: Atoms share electrons. Example: In water (H₂O), oxygen shares electrons with two hydrogens.
Ionic bonds: One atom gives an electron to another. Example: In table salt (NaCl), sodium gives one electron to chlorine.
Metallic bonds: Electrons move freely among atoms, which is why metals conduct electricity.
These bonds explain almost all of chemistry, from why sugar dissolves in tea to how gasoline burns in a car.
A Short History of the Atom
The idea of atoms isn’t new. Over 2,000 years ago, a Greek thinker named Democritus suggested that matter is made of tiny indivisible particles. For centuries it was just a theory.
In the 1800s, John Dalton created the first scientific atomic model. Later, discoveries by J.J. Thomson (electrons), Ernest Rutherford (the nucleus), and Niels Bohr (electron shells) built the picture we use today. Modern science even shows that protons and neutrons are made of smaller things called quarks!
Atoms in Space and Life
Atoms aren’t just important in classrooms they are the key to understanding the universe. Inside stars, small atoms like hydrogen fuse together to make bigger ones like helium. This process, called nuclear fusion, gives stars their energy.
When massive stars explode as supernovae, they scatter heavier atoms like carbon, calcium, and iron into space. Those atoms later formed planets, oceans, and even living things. In fact, the iron in your blood and the calcium in your bones were created in stars billions of years ago.
Why Atoms Matter
Atoms may be tiny, so small you’d need millions lined up just to cross the width of a hair, but they explain everything about the physical world. By studying atoms, scientists understand electricity, chemical reactions, new materials, and even the origins of life itself.
In Conclusion
An atom is much more than “just a particle.” It’s a miniature system with protons, neutrons, and electrons working together. By combining in different ways, atoms make all the matter in the universe.
So next time you hold a glass of water, remember: it’s not just H₂O. It’s billions of atoms, arranged perfectly to give you one of the most important substances for life.