Artificial sweeteners: structure, sweetness, and metabolism
Artificial sweeteners are substances used to provide sweetness to foods and drinks without adding significant calories or sugar. They are commonly found in diet sodas, sugar-free candies, chewing gum, and even some medicines. Although they taste sweet like sugar, their chemical structure and the way our bodies process them are very different.
So how can something with little or no sugar still taste sweet? The answer lies in chemistry.
What Makes Something Taste Sweet?
Sweetness is detected by special taste receptors on our tongue, mainly the T1R2–T1R3 receptor pair. When a molecule fits into these receptors in the right way, the brain interprets the signal as “sweet.”
Interestingly, a substance does not need to be sugar to activate these receptors. Many artificial sweeteners have structures that interact strongly with sweet receptors, sometimes much more strongly than sugar itself. This is why they can taste hundreds or even thousands of times sweeter than sucrose (table sugar).
Common Artificial Sweeteners and Their Structures
Let’s look at a few widely used artificial sweeteners and how their chemical structures affect sweetness.
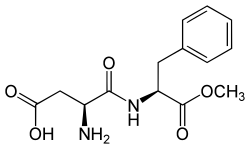
Molecular structure of aspartame
1. Aspartame
Aspartame is made from two amino acids: aspartic acid and phenylalanine, which are building blocks of proteins. Chemically, it is a small peptide with an ester group.
Even though it looks nothing like sugar, its shape allows it to bind well to sweet receptors. Aspartame is about 200 times sweeter than sugar, meaning only a tiny amount is needed.
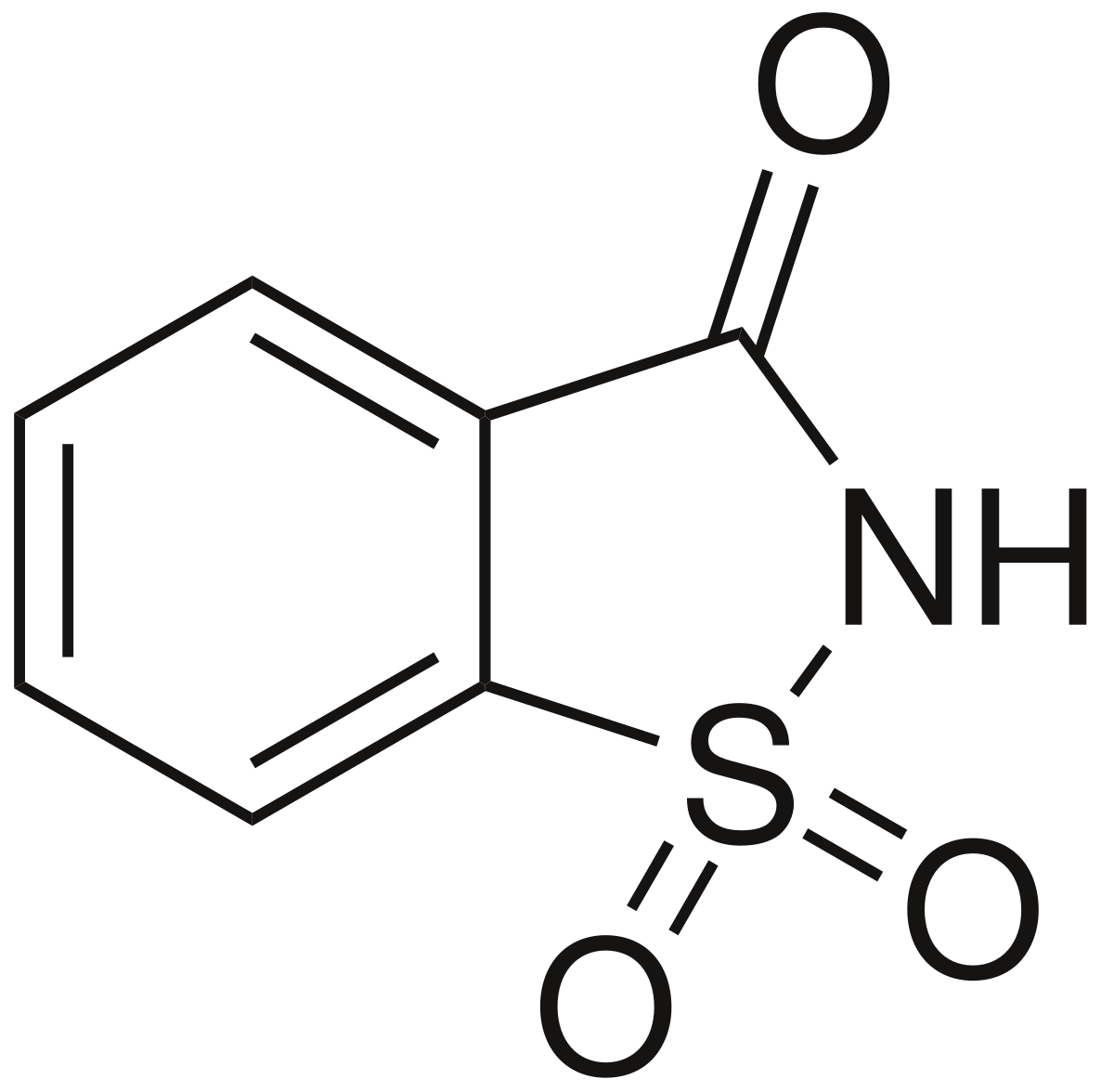
Molecular structure of saccharin
2. Saccharin
Saccharin has a ring-like structure containing sulfur, oxygen, and nitrogen atoms. It does not resemble carbohydrates at all, yet it is 300–400 times sweeter than sugar.
Its rigid structure fits tightly into the sweet receptor, producing a strong signal. However, it can also leave a slightly bitter or metallic aftertaste.
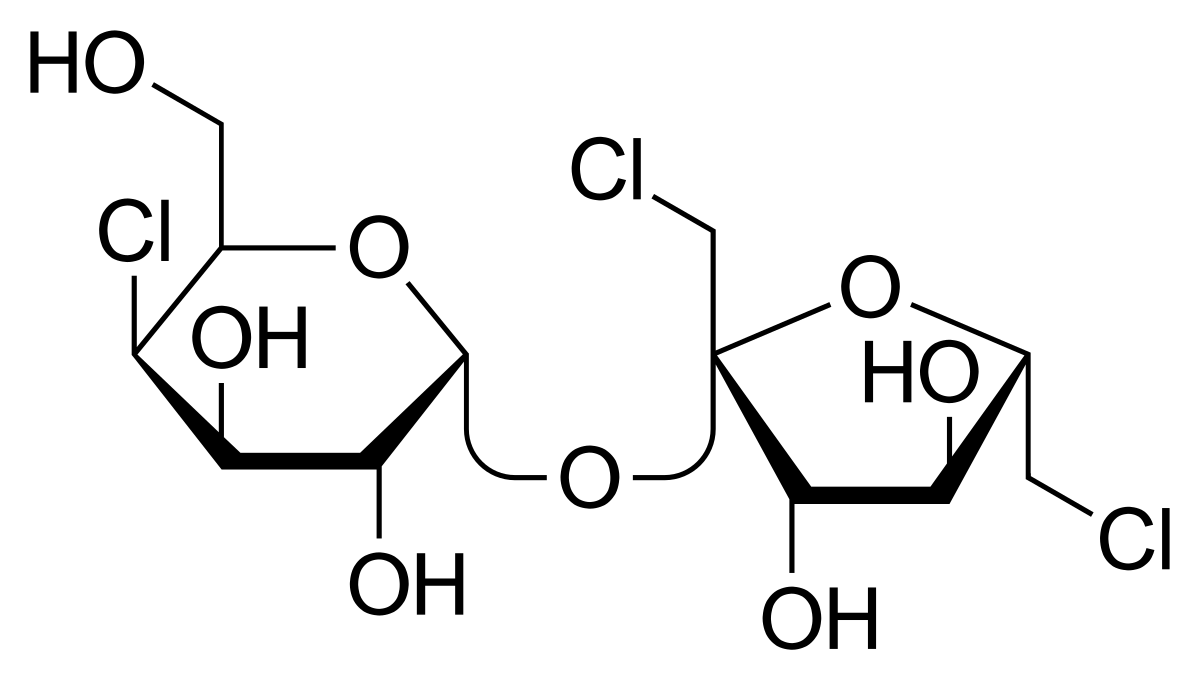
3. Sucralose
Sucralose is chemically similar to sugar, but with a key difference: three hydroxyl (–OH) groups in sucrose are replaced by chlorine atoms.
Molecular structure of sucralose
This small change has a big effect. The chlorine atoms prevent enzymes in our body from breaking it down, while also increasing sweetness. Sucralose is about 600 times sweeter than sugar.
Why Are Artificial Sweeteners So Sweet?
Sweetness depends on how strongly a molecule activates taste receptors, not on how much energy it contains. Artificial sweeteners:
Bind more tightly to sweet receptors
Trigger stronger signals than sugar
Are effective in very small amounts
Because of this, they deliver intense sweetness without needing to be eaten in large quantities.
How the Body Metabolizes Artificial Sweeteners
Metabolism refers to how the body breaks down and uses substances. Artificial sweeteners vary greatly in how they are metabolized.
Aspartame and Metabolism
Aspartame is broken down in the body. Digestive enzymes split it into aspartic acid, phenylalanine, and methanol. These are absorbed and processed like normal nutrients. This is why people with phenylketonuria (PKU) must avoid aspartame, as they cannot safely metabolize phenylalanine.
Saccharin and Sucralose
Saccharin and sucralose are mostly not metabolized. They pass through the digestive system largely unchanged and are excreted in urine or feces. Since they provide little to no energy, they do not significantly raise blood glucose levels.
Are Artificial Sweeteners Safe?
Artificial sweeteners have been extensively studied. Regulatory agencies like the FDA and EFSA set acceptable daily intake (ADI) limits to ensure safety. When consumed within these limits, artificial sweeteners are considered safe for the general population. However, scientists continue to study their long-term effects, especially on gut bacteria and appetite regulation.
In Conclusion
Artificial sweeteners show how chemistry directly affects everyday life. Their unique molecular structures allow them to taste incredibly sweet without being sugar. Some are broken down by the body, while others pass through unchanged. By understanding their structure, sweetness, and metabolism, we can better appreciate how small changes at the molecular level can have a big impact on taste and nutrition.