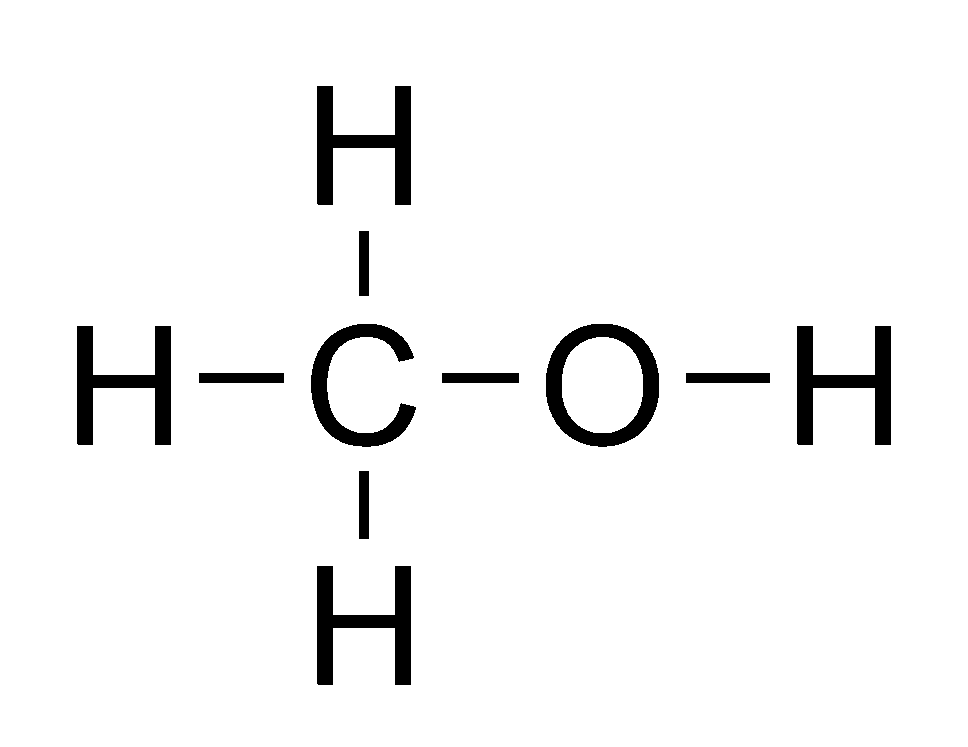
What Is Alcohol?
When most people hear the word alcohol, they think of drinks like beer, wine, or vodka. But in chemistry, alcohol refers to a whole group of organic compounds that contain a hydroxyl group (–OH) bonded to a carbon atom. These substances have very different uses, ranging from fuels and disinfectants to the beverages found at social events.
The most familiar example is ethanol (C₂H₅OH), the type of alcohol in alcoholic drinks. Other alcohols, like methanol (CH₃OH) and isopropanol (C₃H₇OH), are used in industry and medicine but are toxic to humans if consumed.
The Chemical Structure
What makes something an alcohol? The key is the hydroxyl group. Chemists classify alcohols based on the carbon atom attached to that –OH group:
Primary alcohols: the –OH is attached to a carbon connected to only one other carbon (like ethanol).
Secondary alcohols: the carbon with –OH is bonded to two other carbons (like isopropanol).
Tertiary alcohols: the carbon with –OH is attached to three other carbons (like tert-butanol).
Ethanol
This classification helps predict how the alcohol will react in chemical processes.
Common Types of Alcohol
Isopropanol
Methanol
Ethanol (C₂H₅OH)
Found in beer, wine, and spirits.
Also used as a solvent and as a renewable fuel (bioethanol).
Methanol (CH₃OH)
A simple alcohol used in antifreeze and industrial processes.
Highly toxic, just a few milliliters can cause blindness or death.
Isopropanol (C₃H₇OH)
The main ingredient in rubbing alcohol.
Widely used as a disinfectant and cleaning agent.
How Is Ethanol Made?
There are two main ways ethanol is produced:
1.Fermentation
Microorganisms like yeast break down sugars into ethanol and carbon dioxide.
Example:
C6H12O6→2C2H5OH+2CO2C₆H₁₂O₆ → 2C₂H₅OH + 2CO₂C6H12O6→2C2H5OH+2CO2
(Glucose → Ethanol + Carbon Dioxide)This natural process has been used for thousands of years to make beer and wine.
2.Synthetic Production
Ethanol can also be made in factories by hydrating ethene (a petroleum by-product).
Reaction:
C2H4+H2O→C2H5OHC₂H₄ + H₂O → C₂H₅OHC2H4+H2O→C2H5OH
The Effects of Alcohol on the Body
When consumed, ethanol affects the central nervous system. It acts as a depressant, slowing brain activity and reaction times. In small amounts, it may cause relaxation. In larger amounts, it affects coordination, memory, and judgment.
Short-term effects: dizziness, slowed reflexes.
Long-term effects: liver damage, increased risk of cancer, and addiction (alcoholism).
Importantly, other alcohols like methanol and isopropanol are not safe to drink. They are poisonous because the body converts them into harmful substances like formaldehyde and acetone.
Alcohol Beyond Drinking
Chemistry students often find it surprising that alcohols are much more than beverages:
Medicine: Isopropanol is used as a disinfectant. Ethanol is a common solvent in cough syrups.
Industry: Alcohols are raw materials for making plastics and perfumes.
Energy: Bioethanol is added to gasoline as a renewable fuel source.
This versatility is due to the unique balance of alcohols, they dissolve in both water and many organic compounds.
Environmental Considerations
Producing alcohol has environmental impacts. Large-scale fermentation for bioethanol requires crops like corn or sugarcane, raising concerns about land use and food supply. At the same time, alcohol fuels burn more cleanly than fossil fuels, reducing greenhouse gas emissions.
Is Alcohol Always Good or Bad?
Like many chemicals, alcohol is neither purely harmful or purely beneficial. Its impact depends on the type, use, and amount:
Helpful: cleaning wounds, fueling engines, and preserving medicines.
Harmful: when abused as a beverage or accidentally ingested in toxic forms like methanol.
For humans, moderate ethanol consumption may be socially accepted, but health experts emphasize caution, especially for teenagers, since the developing brain is more vulnerable to alcohol’s effects.
In Conclusion
Alcohol is much more than just a drink, it’s a whole family of chemical compounds with important roles in medicine, industry, and energy. From yeast fermenting sugars to chemists synthesizing fuels, alcohols show up everywhere in daily life.
Understanding their chemistry helps us see why ethanol is safe in moderation but methanol and isopropanol are dangerous. It also highlights alcohol’s broader role in shaping human culture, technology, and even the future of sustainable energy.